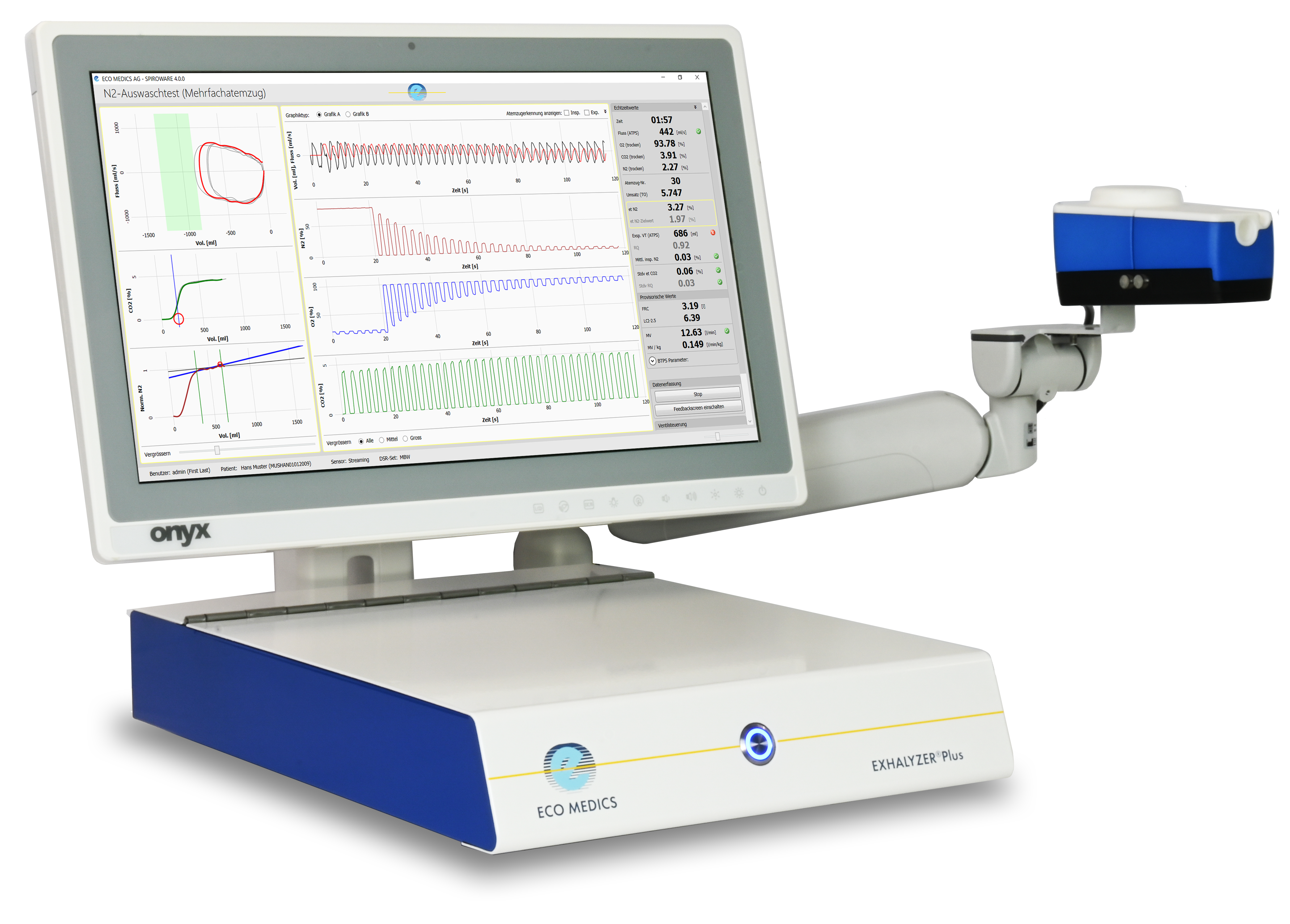
EXHALYZER®Plus
Are you interested? Contact us for more information.
Experience a New Way to Measure
The EXHALYZER®Plus combines LCI testing and spirometry in a single device based on renowned technology. With automatic gas calibration and verification by the SmartBypass system, reproducible and accurate measurements can be initiated without any hardware adjustments – saving valuable time for the operator. The adjustable, ergonomic arm enhances comfort and ease of use for both patients and clinicians, delivering a smooth testing experience from start to finish.
Accurate Results
The EXHALYZER®Plus fulfills pulmonary function standards, which have been validated in scientific publications. Built-in quality control features help clinicians maintain consistent accuracy with every measurement. In addition, aerodynamically optimized flow modules ensure high-precision flow measurement for accurate results.
Comprehensive PFT Software
SPIROWARE® software supports the entire testing process – from patient data entry and test recording to result analysis and reporting – all within a single, intuitive platform. Clear step-by-step instructions guide clinicians through setup and test procedure, ensuring smooth operation at every stage. Robust data security features, including encryption, access control, and detailed log files, help to protect sensitive information and support regulatory compliance. SPIROWARE® software can be adapted to your needs: Test procedures can be easily customized, from practical simplification to scientific requirements. Display options include numerical and graphical data analysis. Respiratory conditions can be monitored over time and displayed in a trend curve.
Solid compliance
The EXHALYZER®Plus and SPIROWARE® software are fully compliant to the recent ATS / ERS recommendations for pulmonary function testing.
® EXHALYZER and SPIROWARE are registered trademarks of ECO MEDICS.
| Flow Measurement Module FlexiFlow (option) |
Prinicple: Ultrasound Sampling frequency: 200 Hz Flow range: ± 1.5 L/s Volume accuracy: ± 2 % or 7 mL (whichever is greater) |
|
| Flow Measurement Module SpiroFlow (option) |
Prinicple: Ultrasound Sampling frequency: 200 Hz Flow range: -10 L/s to + 18 L/s Volume accuracy: ± 2 % or 13 mL (whichever is greater) |
|
| Gas supply (option) | Principle: Open Bypass System (no demand valve) Bypass flow: 1000 mL/s |
|
| CO2 measurement (option) | Principle: Mainstream, single beam infrared Measurement range: 0 to 6 % Accuracy: ± 0.3 % |
|
| Oxygen measurement (option) | Principle: Side stream, laser diode absorption Measurement range: 10 to 100% Accuracy: ± 0.3 % |
|
| Operating specifications | Permitted ambient temperature: 10-40 °C Permitted humidity range: 20-90 % rel. humidity (non-condensing) Atmospheric pressure: 700 – 1100 hPa (max. 3000 masl) |
ECO MEDICS reserves the right to change these specifications without notice.
Applications
- Nitrogen Mutltiple Breath Washout (LCI-Testing)
Assessment of ventilation inhomogeneity and early lung damage - Extended Multiple Breath Washout
Determination of all static lung volumes incl TLC - Nitrogen Single Breath Washout (Slope 3 analysis, SnIII)
Assessment of small airway disease and ventilation inhomogeneity - Spirometry (FVC, FEV1)
General lung condition - Volumetric capnography
- Oximetry
- Tidal breathing analysis (Flow-volume loops)
Early detection of airway obstruction
Your Advantage
- Integrated system for pulmonary function testing suitable for subjects >4 years of age
- Non-invasive detection of lung health
- MDR approval in progress (Medical Device Regulation (EU) 2017/745)
- Compliance with all ATS/ERS recommendations for pulmonary function testing
INTERESTED IN THIS PRODUCT?
References
- Robinson, P. D. et al. Consensus statement for inert gas washout measurement using multiple- and single- breath tests. European Respiratory Journal 41, 507–522 (2013).
- Robinson, P. D. et al. Preschool Multiple-Breath Washout Testing. An Official American Thoracic Society Technical Statement. Am J Respir Crit Care Med 197, e1–e19 (2018).
- Singer, F., Houltz, B., Latzin, P., Robinson, P. & Gustafsson, P. A realistic validation study of a new nitrogen multiple-breath washout system. PLoS ONE 7, e36083 (2012).
- Graham BL, Steenbruggen I, Miller MR, et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med 2019; 200: e70–e88.
